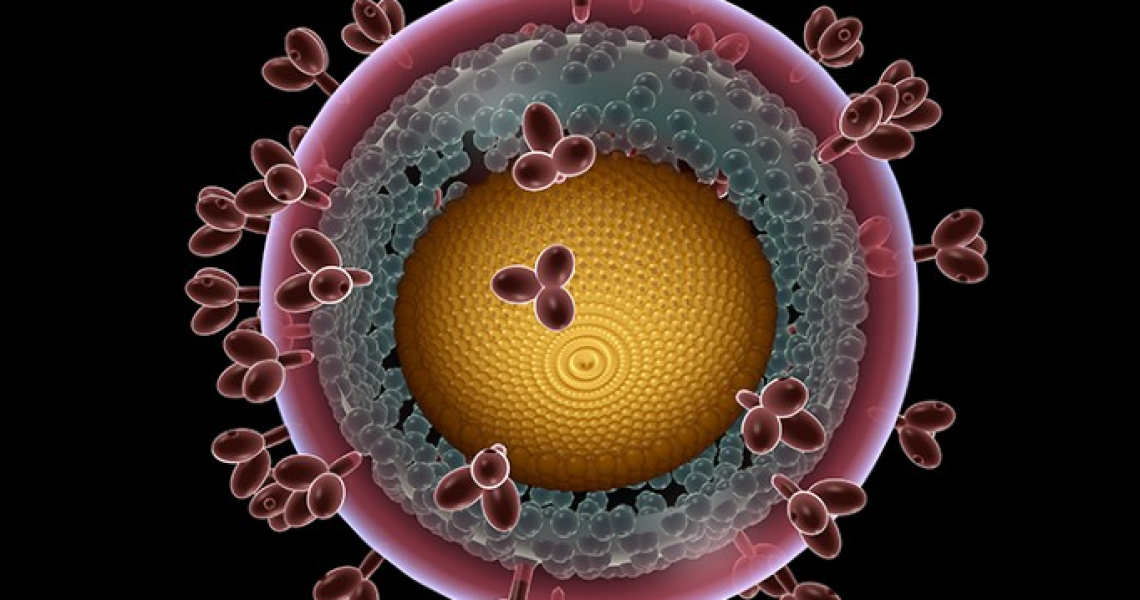
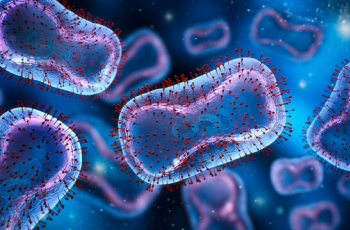
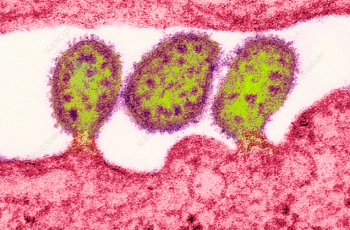
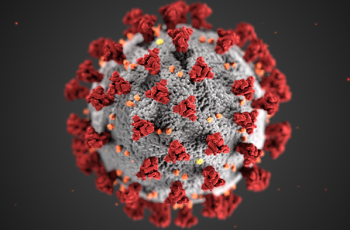
The GW VRU participated in a phase 1 human clinical trial for a recombinant HIV vaccine. This study has promising results and provides proof-of-concept for Env trimer-based GT approaches to activate broadly neutralizing antibody (bnAb) precursors and induce affinity maturation on the path toward mature bnAbs. The structural analyses demonstrate that precise targeting of bnAb precursors with atomic-level accuracy can now be achieved, heralding a new era of vaccine design.
(image from Alamy Stock Photo)